Analysis
Service
iPSC Cell Line Production iPSC Quality Control
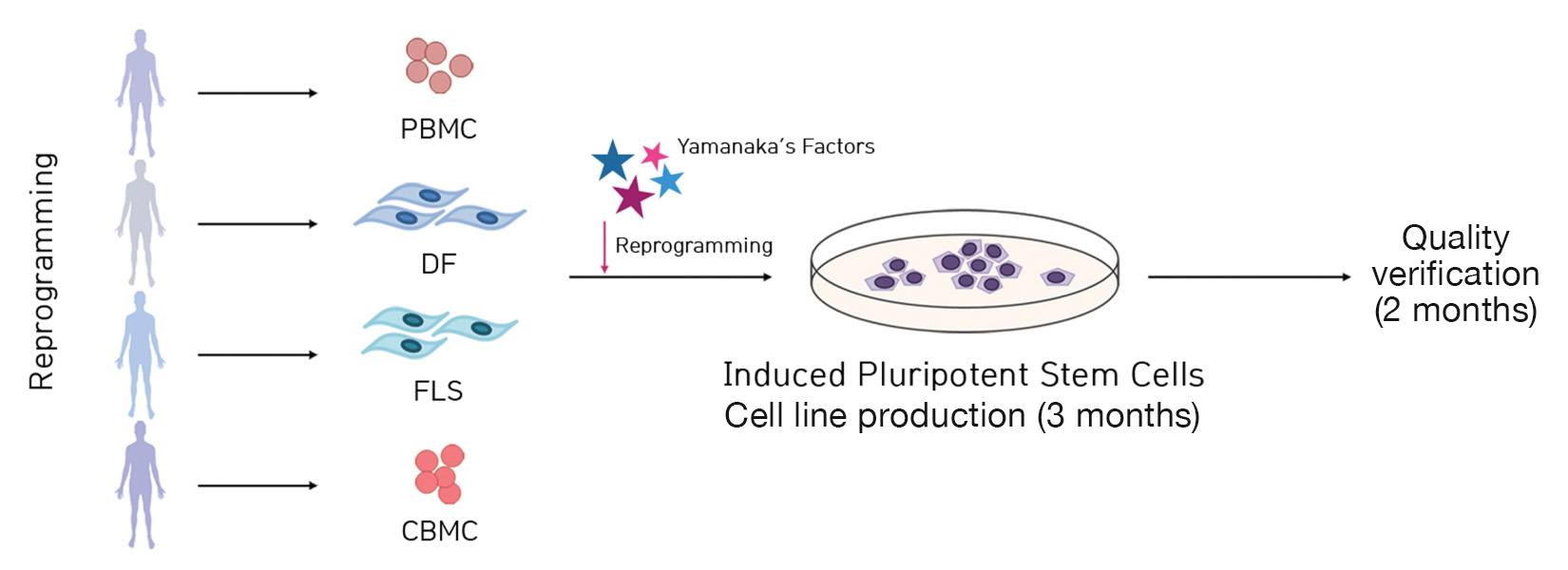
Outline and production lead-time guide
It takes about 3 months to generate induced pluripotent stem cells after receiving primary cells from the client. For quality control, it takes about two months after the induction of iPSCs. Hence, it takes about five months for both iPSC production and quality control if requested at once.
We can produce iPSCs derived from healthy individuals and from patients. In addition to cell line productions, we capable of providing various quality verification services for iPSCs.
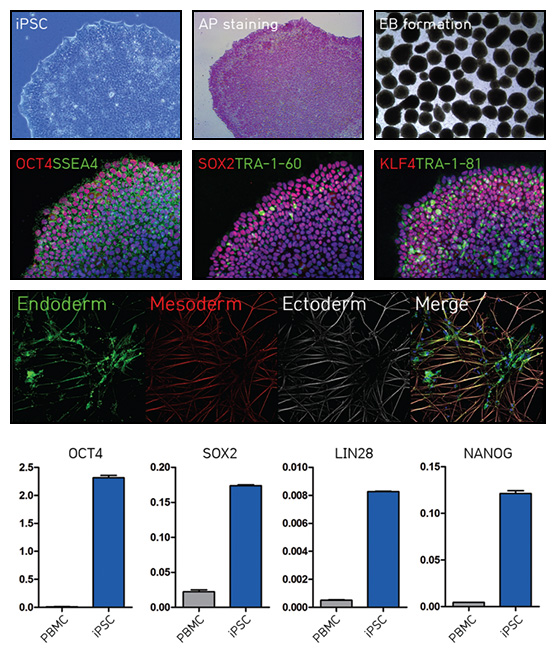
- Alkaline Phosphatase (AP) Staining
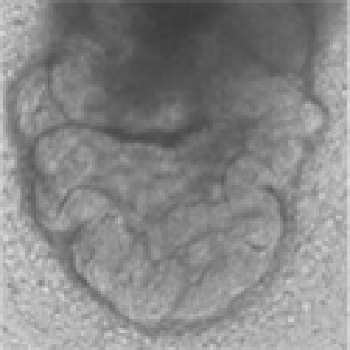
- Embryonic body (EB) Formation
- Gene Expression Verification (qRT-PCR) - OCT4, SOX2, LIN28, NANOG
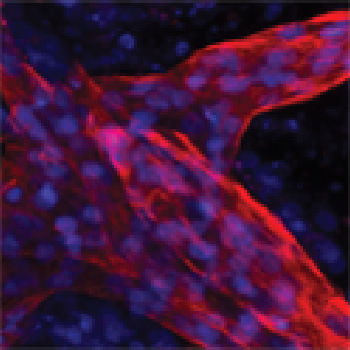
- Immunofluorescence Assay - OCT4, SSEA4, SOX2, TRA-1-60, KLF4, TRA-1-81
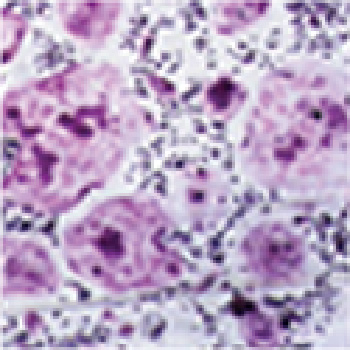
- Tridermic Differentiation Ability Confirmation
iPSC Disease Model
※ If you would like to consult disease modeling using iPSCs,
please contact us via email (support@yipscell.com).
Request for disease modeling analysis service can be submitted via e-mail.
(Required information: disease name, desired analysis experiment, referral drug information)
Disease modeling using iPSC (In-Vitro Efficacy/Toxicology)
We provide drug efficacy evaluation service that can differentiate into various cells suitable for disease using iPSC.
Services available
- Gene Analysis
- Tissue Analysis :
Cardiomyocyte, Intestine, Chondrocyte, Osteoclast, Osteoblast, Hepatocyte, Fibroblast, Motor neuron, Etc.
- Available in both 2-D and 3-D modeling
※ Click each image at the bottom to see detailed explanations
Online Service Application
If you would like to receive our iPSC production and analysis service, please fill out the application form.
Application for induced pluripotent stem cell line production
* This is required